Immunology continues to be one of the most active areas for drug research, since the field aims to bring new ways to eliminate antibodies that drive autoimmune disease, but in a way that also does not compromise the immune system. Merida Biosciences, a startup formed by the Risk Capital firm Third Rock Ventures, emerged from Ski Tuesday, revealing $ 121 million in finance and a main program for a common autoimmune condition that has not had a new therapy approved in decades.
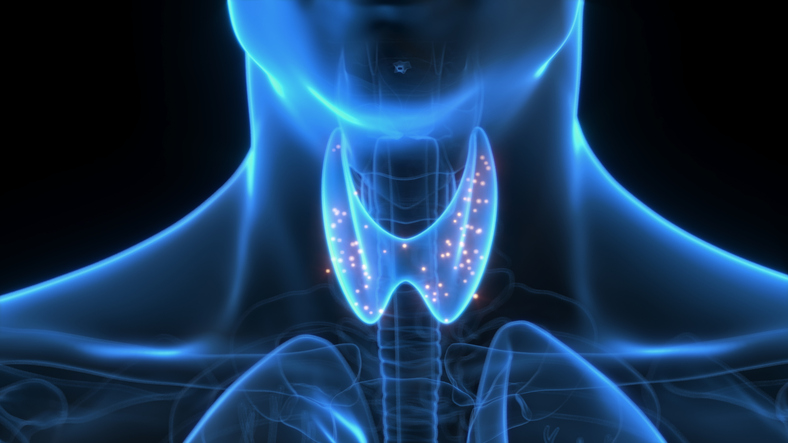
The objective of Cambridge’s main disease, Mérida, based in Massachusetts, is Graves’ disease, which develops as antibodies produced by the immune system attacks the thyroid gland, a butterfly gland located in the front of the neck. Thyroid hormone regulates functions such as metabolism and growth. In Graves’ disease, the attack on the gland results in a thyroid hormone production that accelerates heart rate and weak bones among other problems.
Standard serious treatment includes radioactive iodine therapy to reduce the gland, antithyroid medications or beta blockers that block the effect of thyroid hormone on the body. Each of these treatments introduces risks of complication in other parts of the body. The surgical elimination of the thyroid gland is another treatment option, but patients need hormonal replacement therapy for life later.
Mérida is developing drugs designed to selectively join FC receptors, receptors in immune cells that regulate the immune response. The company says that its protein engineering platform generates unique FC therapies that selectively bind pathogenic antibodies. Then, medications lead to the elimination of thesis antibodies by Hiverbuilt-in cellular mechanisms to clean the antibody complexes, which are molecular structures formed when an antibody joins its specific target antigen. The company says that its medications are designed to eliminate only pathogenic antibodies, saving healthy components of the immune system. In Graves’ disease, this approach is intended to avoid a healthy thyroid signaling.
Mérida medications are biotherapeutics that startup describes as “as antibodies” in the way they move through the body. For example, thesis drugs could provide more durable effects that allow a less frequent dose compared to some other therapies. The Graves program is currently under preclinical research that could support a new research application in research. Other revealed Mérida programs are in preclinical development for primary membranous nephropathy, which is a chronic inflammatory renal disease and food allergies mediated by immunoglobulin E -immunoglobulin antibodies.
“In the heart of Mérida, the mechanisms behind antibody -driven diseases such as tombs at the molecular level, and the hepatic thesis ideas to develop precision therapeutics that until now have evaded the field”, Mérida Gutitaze Saodde and Sausy Sayder and Chiefiodiodies declare. “Our highly differentiated approach has the potential to produce the best treatments in its class by pursuing the fundamental causes of devastating thesis diseases.”
The launch of Mérida occurs when other biotechnology companies centered on immunology, including new companies, advance with their own selective FC receiver aimed at drugs. Roivant Sciences Subidiary Immunovant is looking for several immunological indications with IMVT-1402 and Batoclimab. Both medications candidates are monoclonal antibodies. The IMVT-1402 clinical program includes a 2/3 phase in led in Graves’. Batoclimab is currently in phase 2 tests in Graves’. Last September, Immunovant reported that the preliminary data showing that the Batoclimab achieved a strong response and a shot of antibody levels associated with the disease. The study is expected to publish additional data this summer.
Zenas Biofarma is developing bifunctional antibodies for immunology and inflammatory indications. The Obexelimab lead program is designed to join the CD19 and FC GAMMA IIB receiver, both objectives expressed in B cells. A phase 3 test is being carried out in the disease related to G4 immunoglobulin, backed by an opi of $ 225 million last September. Meanwhile, the Nuvig Therapeutics start is developing proteins designed to point to FC receptors. At the end of last year, Nuvig raised $ 161 million to support clinical development in the middle of the stage of its main program, a potential treatment for the rare autoimmune condition of demyelinizing polyneuropathy of chronic inflammation.
Mérida received Third Rock seed financing in 2022, when Gutierrez was a residence entrepreneur in the company. The finance of the series A announced on Tuesday was directed by Bain Capital Life Sciences, BVF Partners and Third Rock. The other investors in the new round are GV and perceptual risk funds of Xontogeny. The startup is directed by the CEO Adam Towsend, who was the most recent operations director of Pharmaceuticals, a company that develops medications that address the complement system, a part of the immune system.
Illustration: Photo Library of Sebastian Kaulitzki/Science, through Getty Images